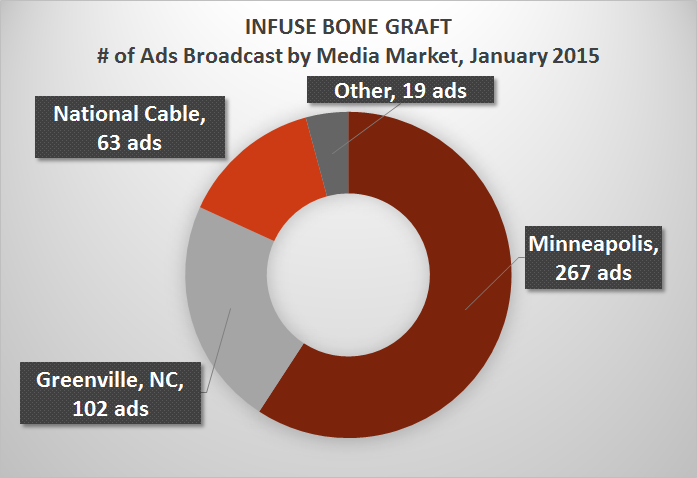
The majority of the Infuse Bone Graft ads in January aired on local broadcast TV channels in the Minneapolis media market. The Brown & Crouppen law firm spent nearly $27,000 to air 267 ads there.
The Balkin Law Group spent more on Infuse Bone Graft ads in January than any other firm -- devoting more than $30,000 to ads aired predominantly on national cable channels.
| | |
January 2015 Infuse Bone Graft Ads: Brown & Crouppen (left); Balkin Law Group (right)
In May, Medtronic, the manufacturer of the Infuse Bone Graft, announced that it would pay $22 million to end product liability lawsuits involving 950 users of the product and that nearly 4,000 additional claims could create settlement costs of $120 to $140 million.
This announcement followed Medtronic’s 2012 agreement to pay $85 million to settle a shareholder lawsuit accusing it of making misleading statements concerning Infuse, a genetically engineered bone graft used in spinal surgery.
As reported at the time, the settlement resolved claims that Medtronic failed to reveal that as much as 85.2 percent of Infuse sales depended on so-called "off-label" uses, where doctors sometimes paid by Medtronic would prescribe the product for applications not approved by the U.S. Food and Drug Administration.
The claimants in the product liability suits allege that the Infuse bone graft caused injury after being used in ways that were not approved by the US Food and Drug Administration.
Shortly the announcement of the settlement in May, the company was sued by Humana Inc. for allegedly paying doctors hundreds or millions of dollars to tout the safety and effectiveness of the Infuse product for a variety of spinal surgeries even though the U.S. Food and Drug Administration had limited its use to lower-back procedures.
More recently, in October, a Maryland appeals court revived a man’s injury suit against Medtronic over the off-label use of its Infuse bone graft device in his surgery, finding that federal law doesn’t preempt his claims about misrepresentations to doctors or the public about the risks of such uses.
As reported by Bloomberg, The Infuse Bone Graft accounted for $471 million in sales in fiscal year 2014, according to a company earnings report.
Studies have shown that Infuse, which helps help bones heal after spinal surgery, has been found to pose an increased risk of cancer along with infections and male sterility. FDA officials warned surgeons not to use the product in cervical-spine procedures in July 2008 after learning of complications in dozens of patients.
This announcement followed Medtronic’s 2012 agreement to pay $85 million to settle a shareholder lawsuit accusing it of making misleading statements concerning Infuse, a genetically engineered bone graft used in spinal surgery.
As reported at the time, the settlement resolved claims that Medtronic failed to reveal that as much as 85.2 percent of Infuse sales depended on so-called "off-label" uses, where doctors sometimes paid by Medtronic would prescribe the product for applications not approved by the U.S. Food and Drug Administration.
The claimants in the product liability suits allege that the Infuse bone graft caused injury after being used in ways that were not approved by the US Food and Drug Administration.
Shortly the announcement of the settlement in May, the company was sued by Humana Inc. for allegedly paying doctors hundreds or millions of dollars to tout the safety and effectiveness of the Infuse product for a variety of spinal surgeries even though the U.S. Food and Drug Administration had limited its use to lower-back procedures.
More recently, in October, a Maryland appeals court revived a man’s injury suit against Medtronic over the off-label use of its Infuse bone graft device in his surgery, finding that federal law doesn’t preempt his claims about misrepresentations to doctors or the public about the risks of such uses.
As reported by Bloomberg, The Infuse Bone Graft accounted for $471 million in sales in fiscal year 2014, according to a company earnings report.
Studies have shown that Infuse, which helps help bones heal after spinal surgery, has been found to pose an increased risk of cancer along with infections and male sterility. FDA officials warned surgeons not to use the product in cervical-spine procedures in July 2008 after learning of complications in dozens of patients.


 RSS Feed
RSS Feed
